INCI:
In November 2006, it became law to include a list of ingredients on the product or packaging of all cosmetics sold in Canada. INCI stands for International Nomenclature of Cosmetic Ingredients. The INCI system was established in the early 1970’s by the Personal Care Products Council. It is a nomenclature developed for international recognition to identify cosmetic ingredients. It is a mandatory nomenclature in the United States, European Union and Canada.
Listing of Ingredients:
The ingredients in a product are listed in order of predominance with the ingredients used in the highest amount first followed in descending order by those in smaller amounts. Ingredients that are less than 1 % concentration and colouring agents may be listed in random order at the end of the list.
Product Identity:
The product identity declaration is a statement of the product’s common or generic name or it may be defined in terms of its function.
Claims:
Claims for all products must be true and verifiable without exaggerating the results of using the product. The product does not make any claims of having therapeutic properties such as prevention of disease, disorder or abnormal condition.
Directions of Use/Warnings:
Use and warnings/caution are necessary for safe product use and have two purposes:
- to prevent misuse
- to promote safe use
Dealer:
A dealer is the person who is a retailer, manufacturer, processor or producer of a product or a person who is engaged in the business of importing, packing or selling a product. Th principle place of business by or for whom the prepackaged product was manufactured or produced for resale is required on the packaging of the product. The name and address are required for postal delivery and a phone number is optional for consumers to contact for product related inquiries.
English and French Labelling Requirement:
In Canada, all mandatory label information should be in English and French including the net quantity. The only exception applies for the manufacture/distributor information which can appear in either language.
Net Quantity:
The net quantity of the product should be displayed on the front packaging of the product. Generally, the net quantity should be expressed in metric units of volume when the product is a liquid, gas or viscous.
You may also see these symbols on cosmetic products imported form Europe:
 Best Before the End of – any cosmetic product that is good to use less than 30 months
Best Before the End of – any cosmetic product that is good to use less than 30 months
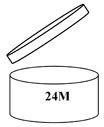 Period After Opening – any cosmetic product that is good to use more than 30 months
Period After Opening – any cosmetic product that is good to use more than 30 months
 Nominal Net Content – “e” mark should be shown if the product is filled according to the “average fill system”. The “average fill system” comes from the legislation of weights and measures in Europe.
Nominal Net Content – “e” mark should be shown if the product is filled according to the “average fill system”. The “average fill system” comes from the legislation of weights and measures in Europe.
 The Green Dot – this symbol signifies that the producer has made a financial contribution towards the recovery and recycling of packaging in Europe
The Green Dot – this symbol signifies that the producer has made a financial contribution towards the recovery and recycling of packaging in Europe
 Leaping Bunny – internationally recognized symbol guaranteeing consumers that no new animal tests were used in the development of the product.
Leaping Bunny – internationally recognized symbol guaranteeing consumers that no new animal tests were used in the development of the product.