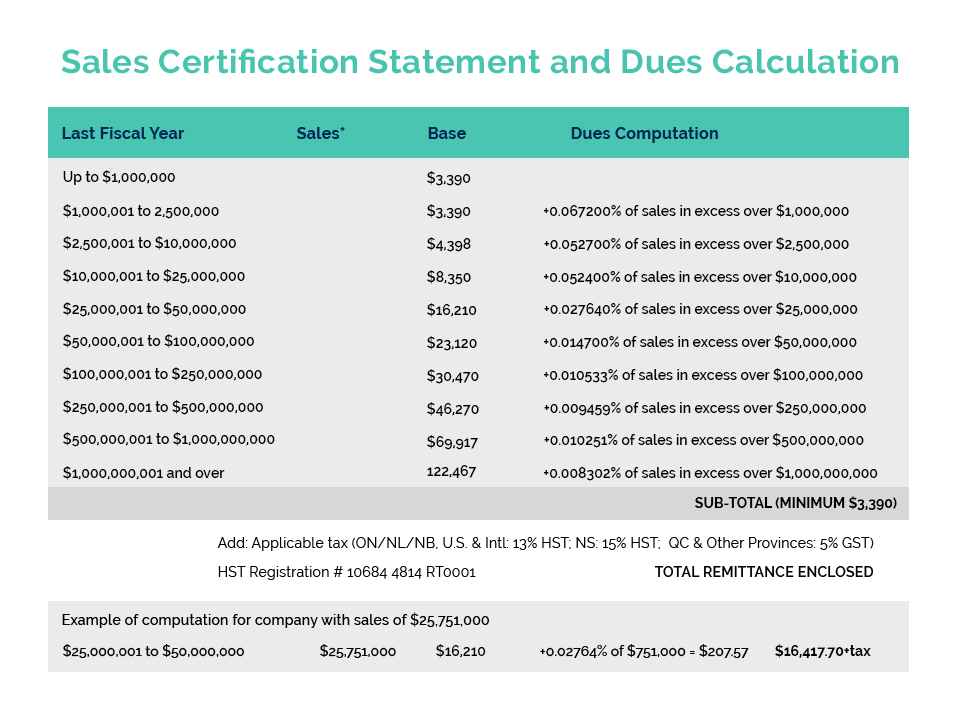
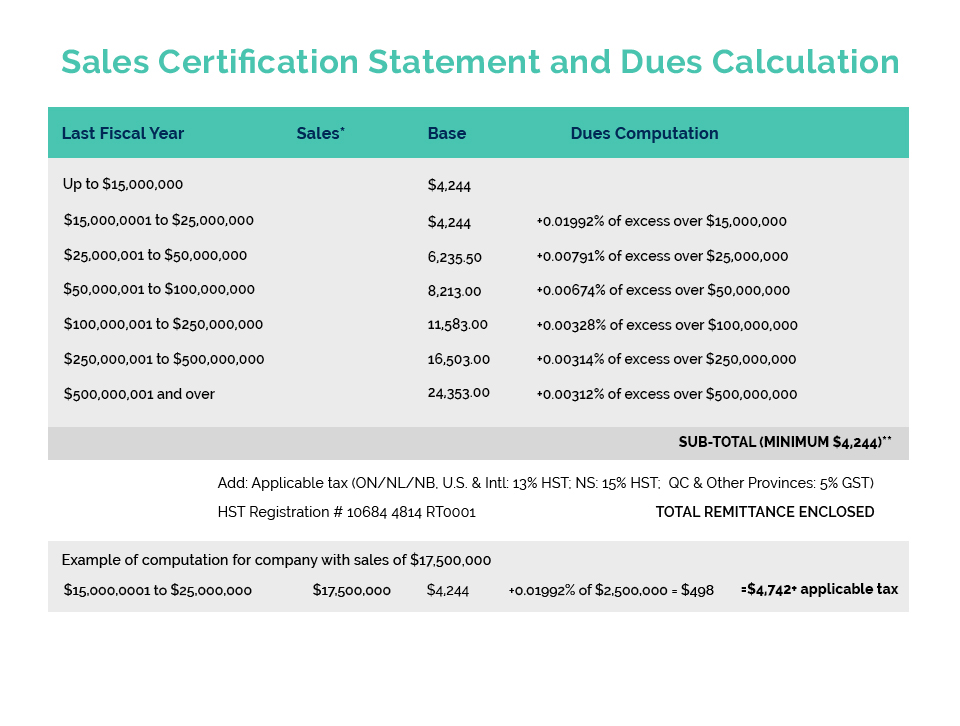
Canadian Custom Packaging
General Position Description:
This position is responsible for a wide range of quality control and control assurance activities relating to raw materials, packaging materials, finished products, line inspection and documentation. Quality assurance personnel must adhere to Good Manufacturing Practices Guidelines (GMP).
Key Duties and Responsibilities:
- Test: raw materials, packaging materials and finished products (focus on packaging materials)
- Calibrate equipment
- Issue lot numbers
- Conduct quality checks
- Operate lab equipment (GC, UV etc.)
- Complete lab documentation (including product release when acting as QAM designate)
- Document methodology
- Prepare samples, Prepare reagents, test solution
- Ensure that laboratory and production equipment are maintained to a standard that is both safe and complies / conforms to all Customer, statutory and regulatory requirements.
- Critically evaluate current procedures and initiate continuous improvements while maintaining an efficient Quality Control operation
- Prepare / modify / maintain SOP’s and related work instruction
- Prepare reports based on results of testing and investigations
- Identify root causes and appropriate preventive actions including documentation of out of spec situations e.g. (production deviations, OOS, change control, product rework)
- Additional duties as assigned by the Manger of Quality Assurance and his/her designate
Primary Microbiologist Back-up – Key Duties and Responsibilities:
- Additional duties as assigned by the Manger of Quality Assurance and his/her designate Assist in Microbiological analysis of raw materials, in-process and finished products as required
- Assists in Media preparation, sterilization and growth promotion for entire microbiology laboratory.
- Evaluate and determine the release of in-process and finished products based on micro results
- Conduct environmental monitoring of plant by microbiological analysis of environmental settle plates and potable water samples
- Interpret and log all data, trend results as required, report deviations
- Organize and manage QA-micro supplies and equipment required to accomplish quality related functions
Knowledge, skills and work experience requirements:
Practical experience in area of responsibility
Familiarity with pharmaceutical regulatory process and QMS, GMP, HPFBI, ISO and GLPKey Competencies:
Proactive, organized: Prioritizes and organizes daily work to meet overall deadlines
Manages own time to meet short term objectives
Analytically minded. Problem solver
Educational requirements:
B.Sc. in Chemistry related field (Biochemistry, Biological Science, etc.)
Must have educational background in microbiology, possess microbiology lab experience (school or work – minimum 1 year preferred).
Post-Graduate Certificate in the Pharmaceutical field considered an asset