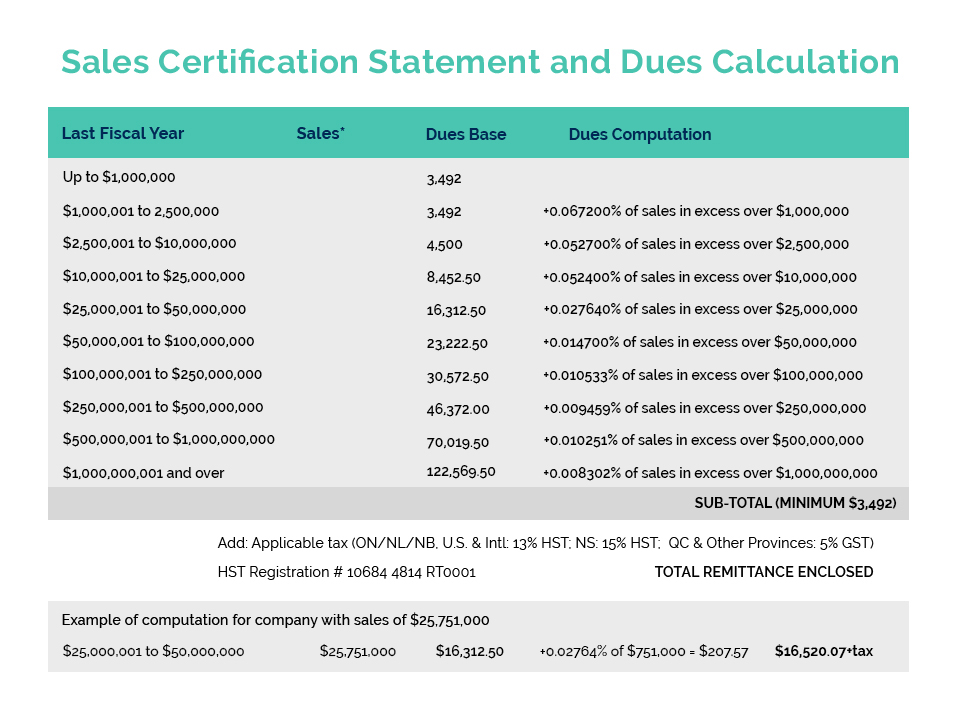
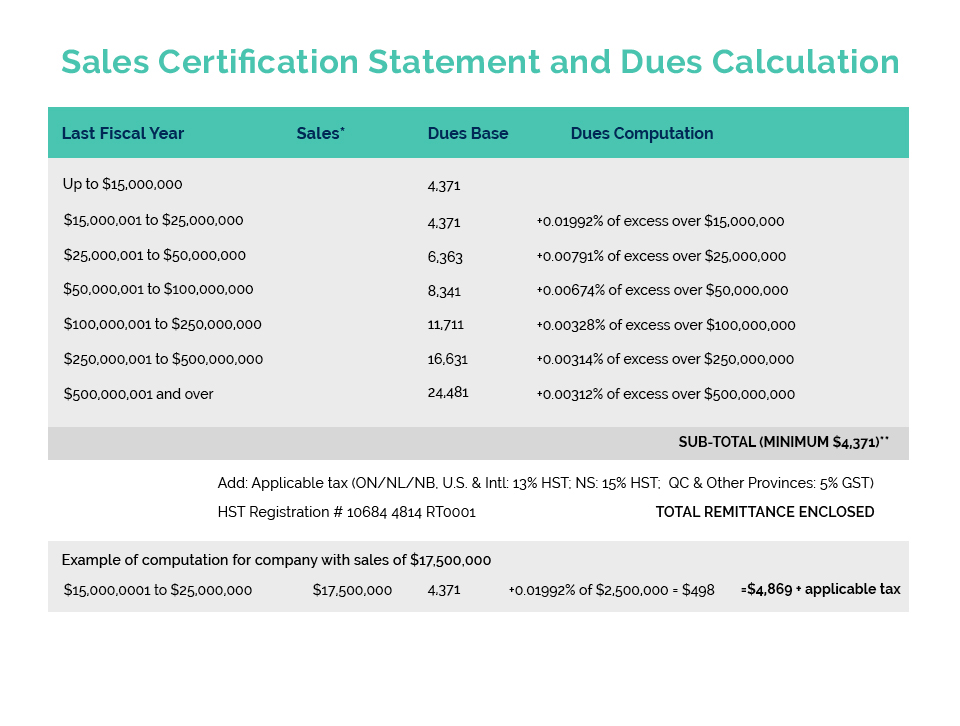
This year’s Spring Regulatory Workshop was held on April 27 in Gatineau, Quebec. Attendees gained critical information and valuable insight from industry and government experts on a variety of issues and topics.
Highlights included:
• Updates on Plain Language Labelling and the Canadian Drug Facts Table
• The path forward for In-Commerce List Substances
• Updates on Cosmetics Alliance service improvements including technical committees and member communications
Speakers presented on the following topics:
• Consumer Product Safety Directorate Updates
• NNHPD Updates & Status of the Self-Care Products Framework
• Regulatory Operations and Regions Branch Updates
• Food and Drugs Act Liaison Office Annual Report and Updates
• Inventory Update
• Cosmetics Alliance Member Engagement Session
As always at our Rgulatory Workshops, members had the opportunity to network with government and industry colleagues which helps to promote continued engagement between our regulatory events.
If you want to learn more about “all things regulatory”, please join us at our next Regulatory Workshop on October 11 at the Holiday Inn Toronto Airport. Mark your calendars now so you don’t miss out on this valuable event. Registration details to follow at a later date.