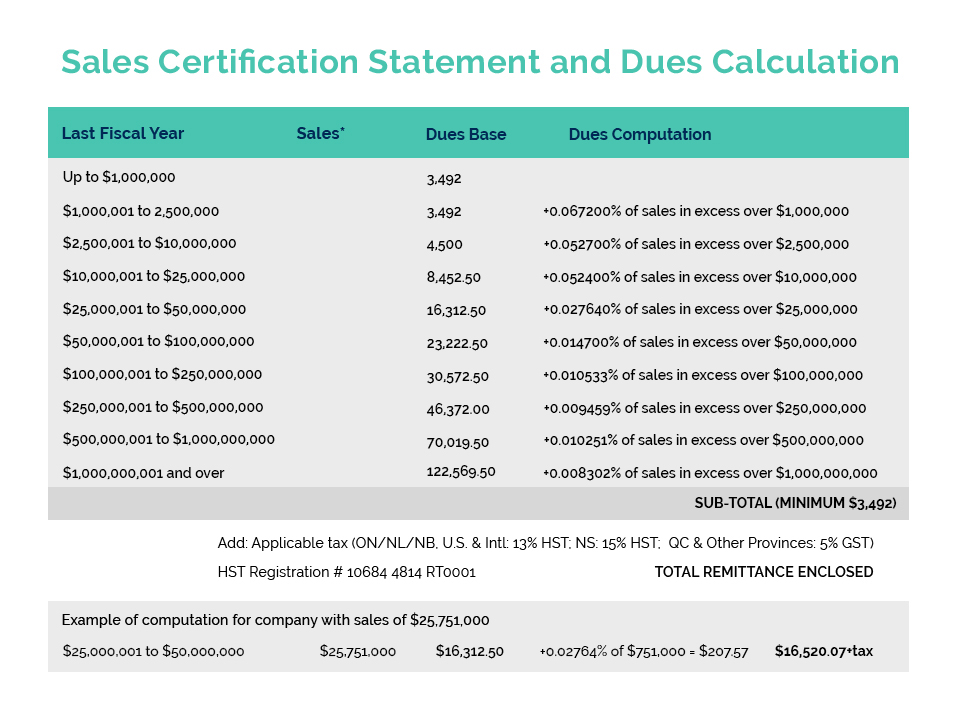
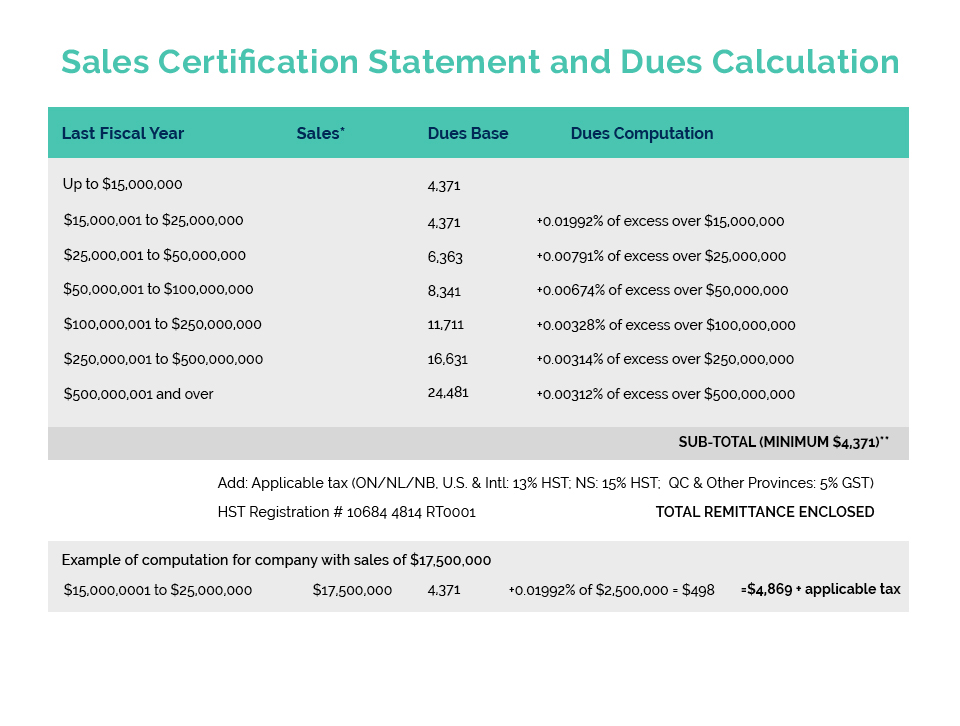
Cost recovery is a federal government policy initiative that allows departments to recoup a portion, or the entirety of costs incurred in delivering a regulatory program. It is intended to ensure that the costs of such regulation are ultimately borne by the consumer of the products or services being regulated, rather than the taxpayer in general.
New fees and performance standards for drugs (all D.I.N. products) and medical devices were implemented on April 1st, 2020 with an annual adjustment (based on the consumer prince index or CPI) taking effect on April 1st, 2021.
CA was actively involved along with other industry associations when cost recovery was recently developed. We were instrumental in ensuring that the regulatory costs for our “low-risk” DIN products did not included higher costs that were required to oversee higher risk drugs. We also continue to make the case that any unnecessary costs related to delays in the implementation of the Self-Care Framework take into account or lead to the introduction of interim administrative measures that would relieve our member companies of these unnecessary burdens.
As part of its accountability to stakeholders, Health Canada has indicated its commitment to transparency and accountability through annual reporting and annual engagement on performance, costs, and program efficiencies. Cosmetics Alliance is fully engaged in the process and, on behalf of members, will focus our efforts/comments on matters related to DIN Application, Annual Right to Sell, and Drug Establishment Licensing fees along with the overarching principles of the cost recovery framework.