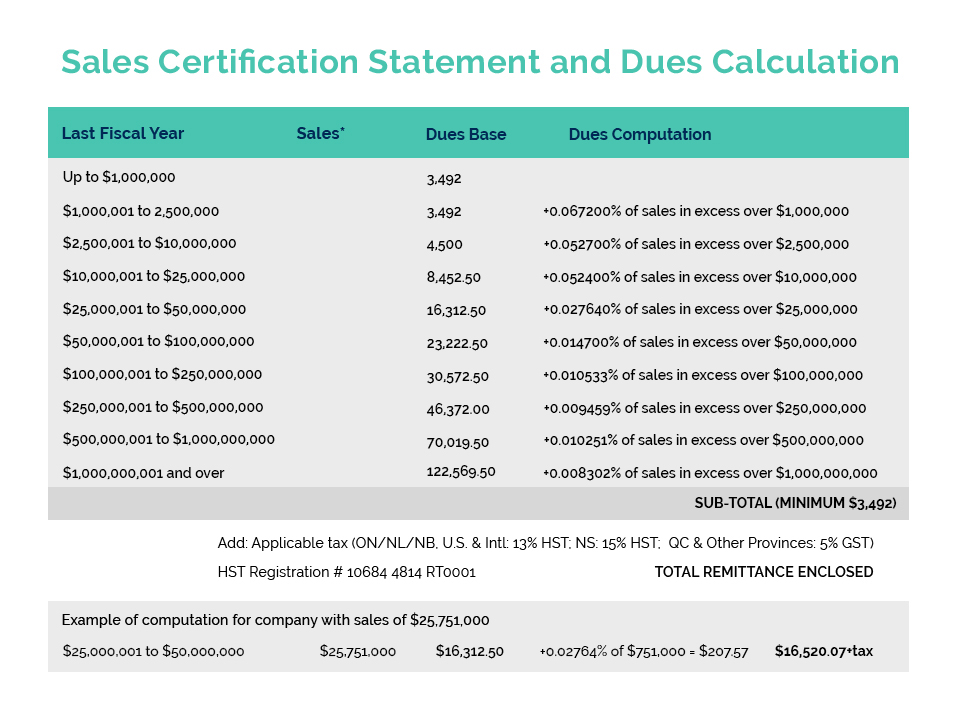
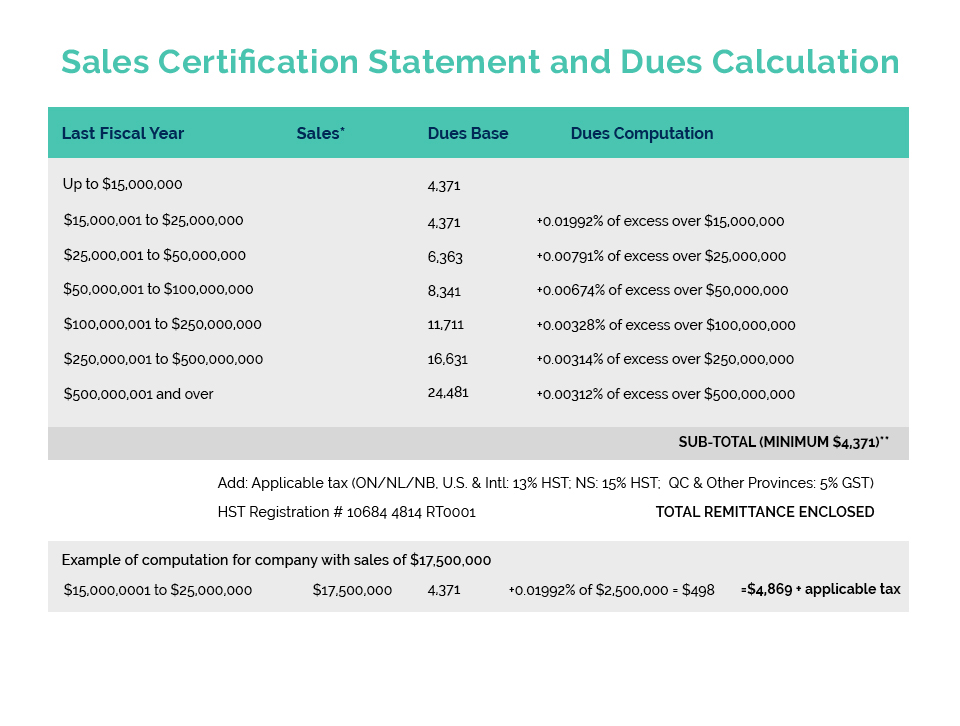
CA has reached out to our federal Ministers responsible for Health and International Trade to formally request the establishment of a new Government issued or authorized GMP compliance certificate to facilitate the export of Canadian manufactured cosmetic products to China.
China’s new cosmetics regulations provide for an exemption to their requirements for animal testing on cosmetic imports. However, the exemption requires that supporting documentation be provided by a “government authority” certifying that the products meet the required GMP/ISO standard for cosmetics.
The issue at hand is that such export certificates are rarely issued by governments for cosmetics and are consequently provided by industry associations including in Canada, the U.S. and many other jurisdictions. This means that without some form of formal “authorization” provided by government, exporters will be unable to take advantage of this new provision and the requirement for unnecessary animal testing will continue.
Cosmetics Alliance is lobbying our federal government to create a new Government issued compliance certificate or provide formal authorization for the certificates already offered by Cosmetics Alliance’s Export Certificates Program that would satisfy Chinese authorities.
Following the issue being raised by the French cosmetics industry and their national trade association FEBEA, it was recently announced by the French National Agency for the Safety of Medicines and Health Products (ANSM)) that they will issue certificates to sites that manufacture ‘ordinary’ cosmetic products. The ANSM has since developed an online platform allowing cosmetic and personal care manufacturers to download the documents necessary to obtain the certificate, allowing all French cosmetics manufacturers to export to China without their products being tested upon arrival.
This development sets a precedent for how we might approach a solution in Canada, as CA has clearly pointed out to our government. If a workable solution can be developed, it would create a significant opportunity for manufacturing cosmetics in Canada. This includes the shifting of production from other facilities to Canadian sites so that products can be exported to China without animal testing requirements.
To this end, CA has requested a meeting with the appropriate officials from Health Canada and Global Affairs – International Trade as cooperation of senior officials from both Ministries is required to advance this opportunity. To assist in our lobby efforts, CA has issued a survey to obtain input from member companies who manufacture cosmetics in Canada and export, or are contemplating export, these products to China. CA has also created a “Letter of Support” template for members to sign and send to government which will strengthen our collective voice on this issue.