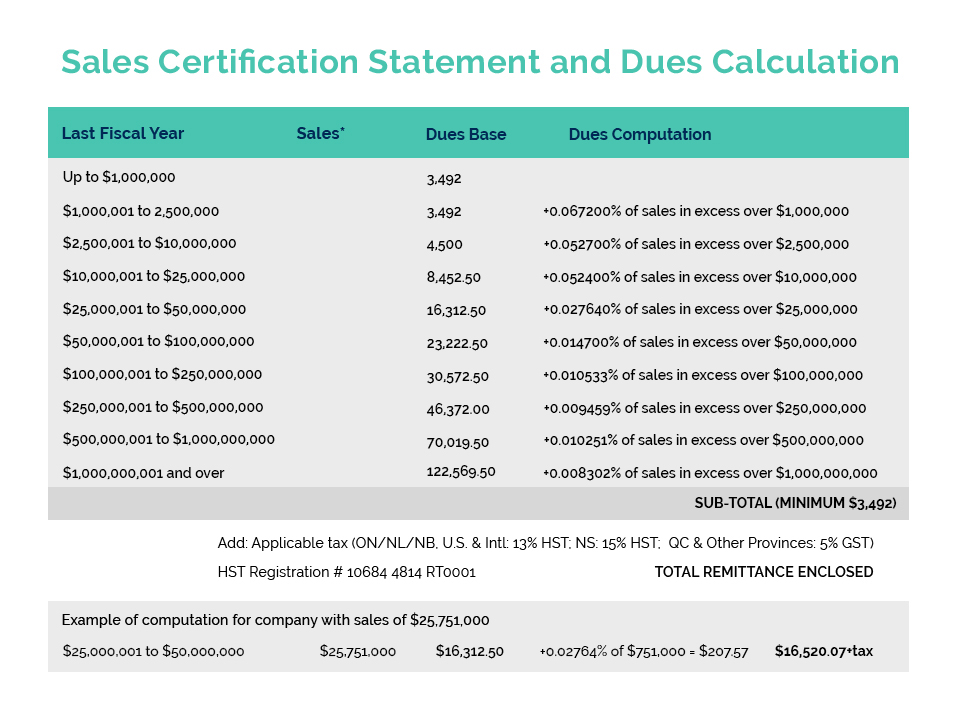
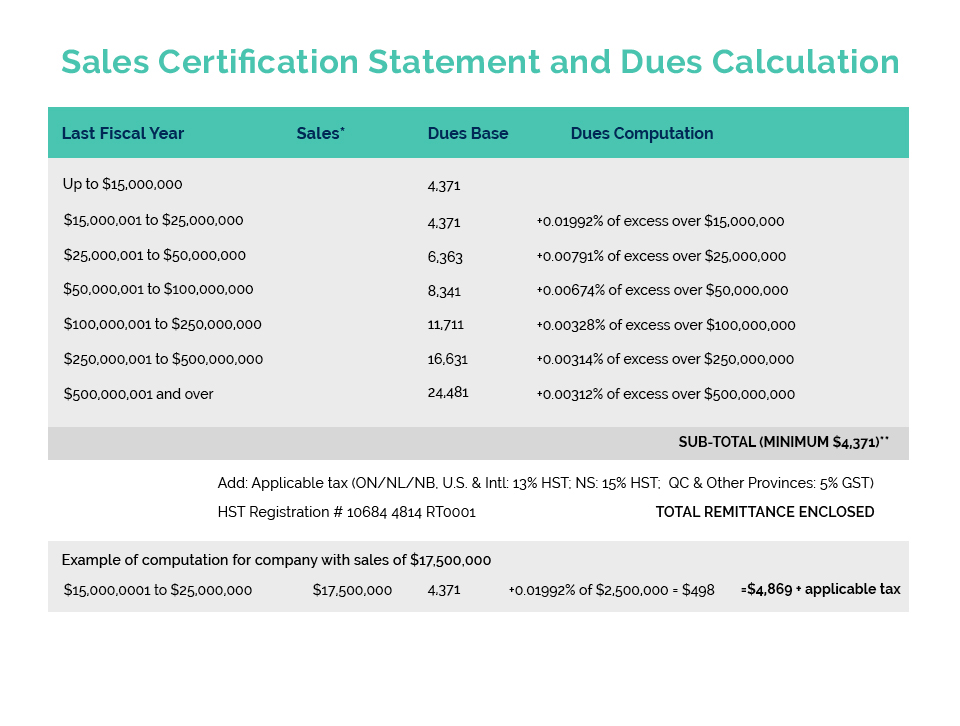
Links of Interest
Health Canada
Health Canada’s Cosmetics Program has the mandate to protect the health of Canadians by minimizing the risk associated with the use of cosmetics marketed in Canada. The Program defines and communicates requirements for the manufacture, labelling, distribution and sale of cosmetics, and evaluates compliance. The basis for the regulatory authority for the Cosmetics Program comes from the Food and Drugs Act.
Environment Canada’s Chemical Management Plan
Ingredients used in cosmetics and other personal care products are regulated under the Canadian Environmental Protection Act. They are also subject to review under Canada’s world-leading Chemicals Management Plan which evaluates the safety of ingredients from both a human and environmental health perspective.
Cosmetic Ingredient Review
CIR was established in 1976 by the U.S. Personal Care Products Council with the support of the U.S. Food & Drug Administration and the Consumer Federation of America. The CIR thoroughly reviews and accesses the safety of ingredients used in cosmetics in an unbiased, expert manner.
Cosmeticsinfo.org
Cosmeticsinfo.org features product & safety information, information on how to read product labels, frequently asked questions and safety information in many product categories. Keep in mind that this website references U.S. cosmetics regulations and not Canadian, where are products are regulated by Health Canada.It is a great source of information about cosmetics and personal care products, their ingredients and how they are tested, which is consistent internationally.
Advertising Standards Canada
ASC is the Canadian advertising industry self-regulatory body. ASC is committed to fostering consumer confidence in advertising through responsible industry self-regulation.