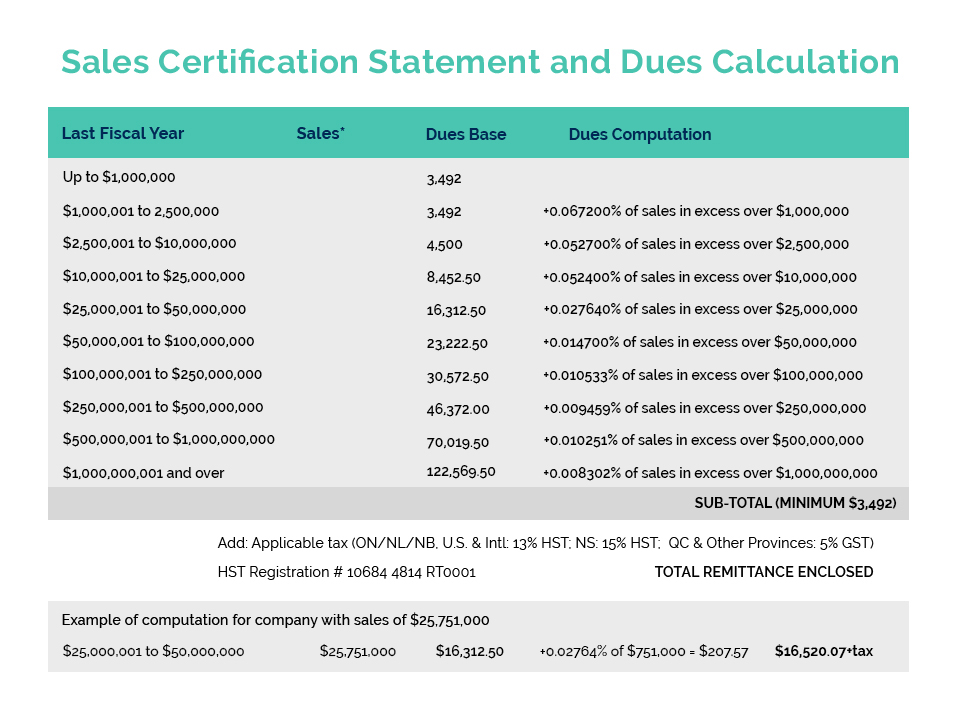
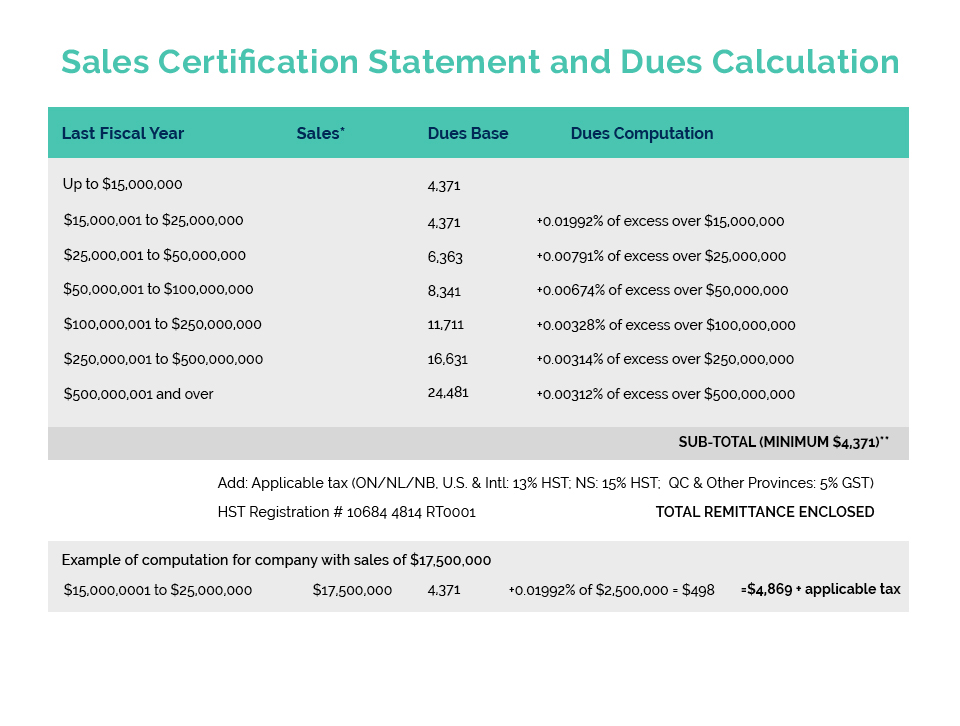
Amendments to China’s cosmetic regulations now provide for an exemption to animal testing requirements for imported products if these products are accompanied by a government issued GMP compliance certificate. Canadian cosmetic manufacturers have been seeking the issuance of such a certificate by Health Canada and in June of last year, CA secured a commitment from then Health Minister Jean-Yves Duclos to establish a joint Health Canada/Industry Task Force to develop options for the Minister on this matter.
The value of cosmetics manufactured in Canada and exported to China is estimated at over $350 million annually. Ensuring they are not subject to animal testing in China is essential to maintain and growing this business.
The Task Force, which includes Dennis Price, Director-General of Health Canada’s Consumer and Hazardous Products Safety Directorate (CHPSD), CA, and several of our member companies, have been working over the past year on the details of an “options paper” that can be presented to new Health Minister Mark Holland.
While in Ottawa last week, CA’s Darren Praznik and Beta Montemayor met with Mr. Price and his team to finalize several details which will be soon presented to the full Task Force for their review and endorsement prior to presentation to the Minister. It is hoped that this cooperative approach established by Minister Duclos last June will be successful in supporting the Canadian cosmetic manufacturing industry. CA will continue to provide updates on further developments.