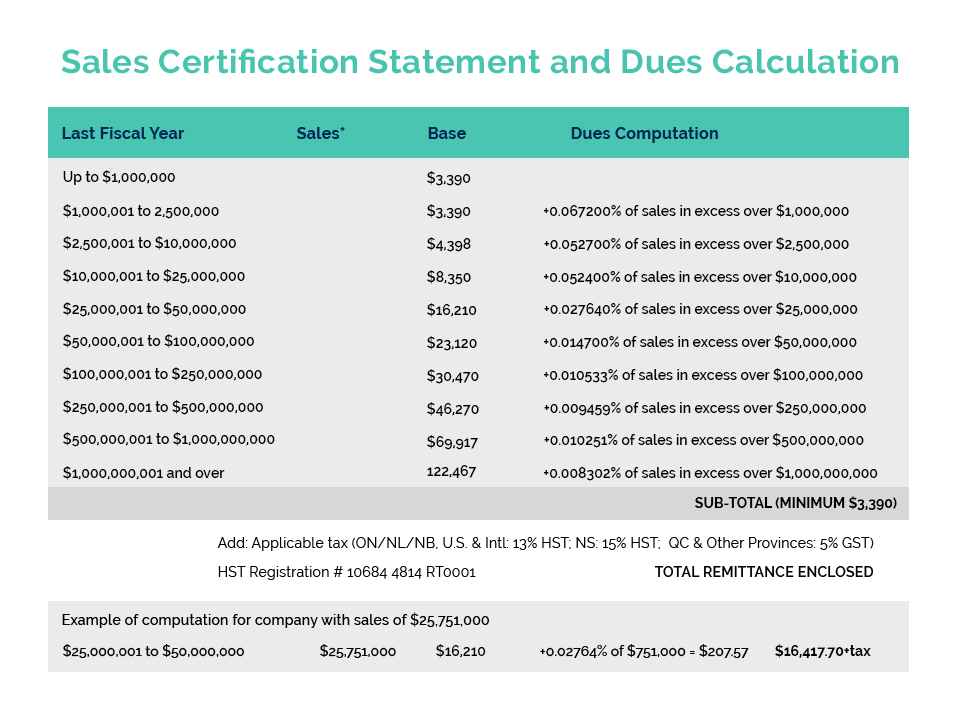
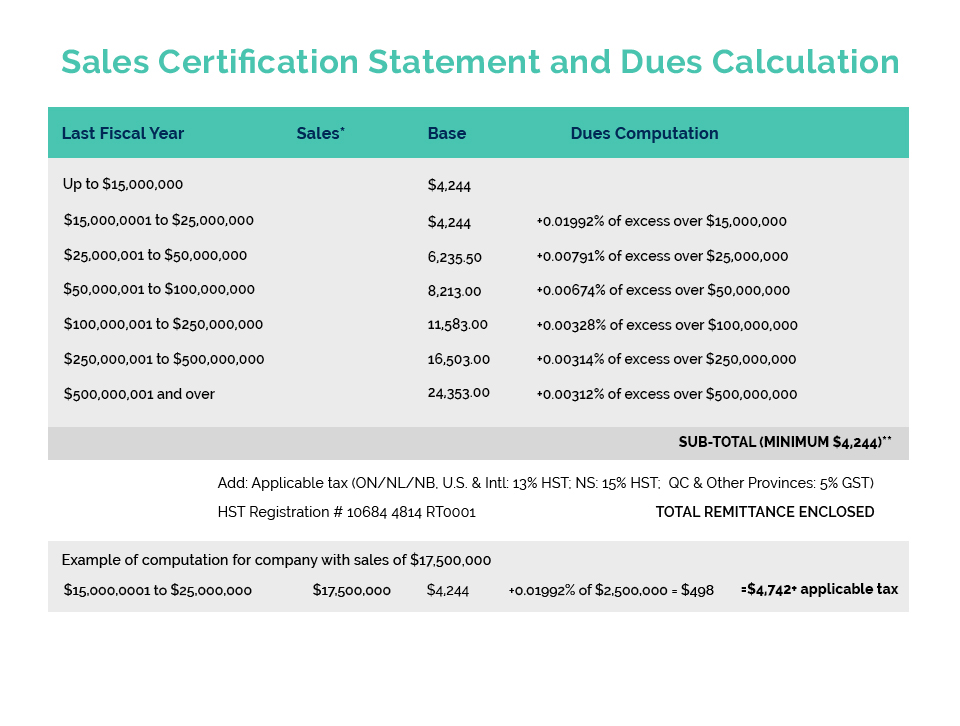
On August 2nd, the SAC-HPCC released their final report and recommendations for Health Canada’s consideration on the safety, efficacy and quality of cannabidiol (CBD) when used for therapeutic purposes.
The mandate was to provide independent scientific and clinical advice to support Health Canada’s consideration of appropriate safety, efficacy and quality standards for health products containing cannabis.
As a next step, Health Canada will be exploring a potential framework for non-prescription health products containing CBD.
Cosmetics Alliance will be working through the report and be providing feedback on the Notice to Stakeholders to help inform the ongoing policy development to support the department’s decision-making via our special Cannabis Task Force. If you are not already member of this task force, please send us an email at regulatory@cosmeticsalliance.ca to learn more details and become a member.
Publication of the Final Report of the Scientific Advisory Committee on Health Products Containing Cannabis
Posted Date: 3-August-2022