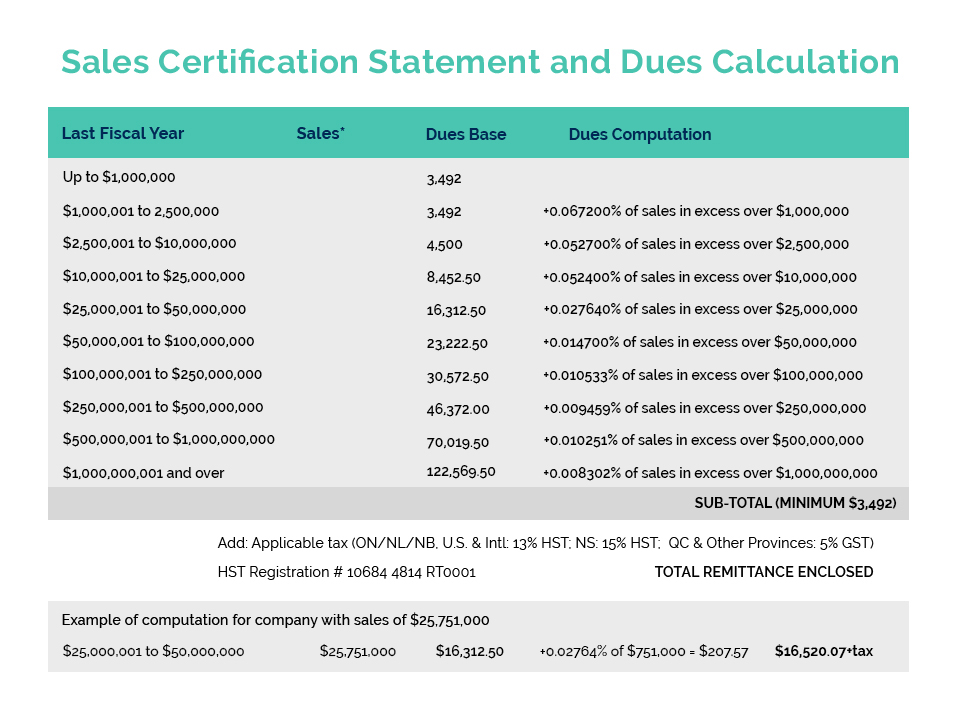
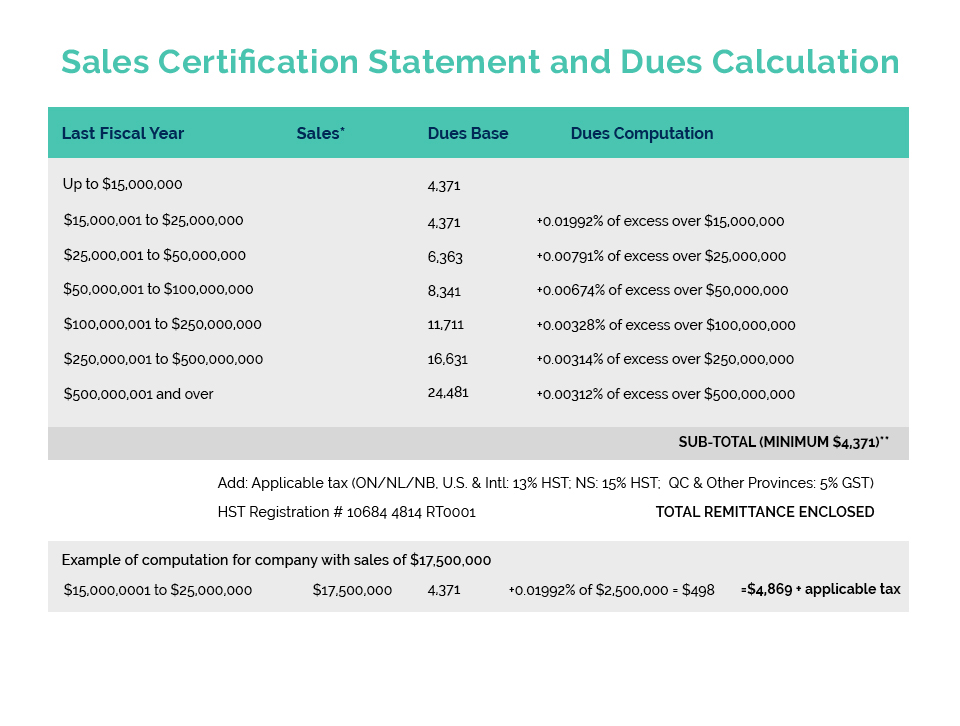
Further to CA’s member update issued May 26, Environment and Climate Change Canada (ECCC) is currently undertaking an “enforcement blitz” with respect to Perfluorononyl Dimethicone – a silicone polymer commonly used in cosmetics to provide a ‘slip and feel’ aesthetic as well as water/sweat-proofing benefits.
AT ISSUE:
Perfluorononyl Dimethicone is a substance subject to a Significant New Activity (SNAc) Notice – 14673 (2007). This SNAc sets out a series of information requirements that must be submitted to authorities PRIOR to the substance being introduced into Canada for specific activities considered to be ‘new’ as identified in the SNAC. In this case, the use/import of this ingredient in a cosmetic/personal care product would be considered a “new activity” for which supplemental data must be notified at least 90 days prior to commencing these activities through the submission of a Significant New Activity Notification (SNAN).
Environment and Climate Change Canada (ECCC) is presently undertaking an enforcement blitz in respect of this SNAc. Their activities appear to be focused on the presence of this substance in certain cosmetic (eye and lip) pencils produced and supplied by Schwan Cosmetics (Germany); although it is probable that activities could extend beyond these specific products, given the nature of the SNAc.
COMPLIANCE CONSIDERATIONS:
Based on supply chain inquiries, including outreach to Schwan Cosmetics (who are not CA members), it appears that there are mitigating circumstances regarding the specific identity and composition of the polymer in question, as well as important clarifications regarding the CASRN originally attributed to this ingredient; in addition to challenges in corresponding communications by the manufacturer and distributor of the implicated raw material.
WHAT COSMETICS ALLIANCE IS DOING:
CA is activity pursuing discussions with senior level officials to “right-size” approaches to compliance on this specific substance and PFAS (more generically) in cosmetics and personal care products. We are actively reminding officials of the current state of knowledge and the present science road map analysis that is in play under Canada’s world-leading Chemicals Management Plan (CMP). We are encouraging officials to remain patient with their compliance approaches while this important planning and scoping exercise under the CMP is being completed. This will then enable a reasonable and proportionate approach to any regulatory concerns related to PFASs.
WHAT MEMBERS SHOULD DO:
- If you import/distribute lip or eye pencils, be aware that you will likely be receiving (if you have not already) a communique from Schwan Cosmetics regarding these developments in Canada. Be on the lookout for this communique as it will provide some important technical clarifications regarding the mitigating circumstances as outlined above.
- Review your procurement/product databases, product labels and/or technical specifications to confirm if you have products entering your Canadian distribution channels containing perfluorononyl dimethicone.
- If yes, you may anticipate (if not already) a visit from ECCC officials who may look to impose an enforcement order in relation to this SNAc
- Regardless of ECCC engagement, CA recommends that you reach out to Schwan Cosmetics (or any other supplier) to review compliance considerations regarding this ingredient
- Send CA a note or schedule a touch-base with us to review possible engagement strategies and next steps (regulatory@cosmeticsalliance.ca with subject line: ECCC PFAS Activity)
ADDITIONAL RECOMMENDATIONS (PFASs in General):
- In addition to specific action on perfluorononyl dimethicone, CA members should take note that ECCC may also consider action on perfluroninated alkyl substance (PFAS) writ large, due to the characterization of these substances as ‘forever chemicals’
- PFASs represent a very large subset of chemistries, spanning potentially into the 1,000’s to many 1,000’s of substances. Although the large majority of these chemicals are unlikely to be directly used by the cosmetics/personal care industry, some of these ingredients may provide significant innovative niche functionality that are critical to product performance.
- Part of the challenge with PFASs is that there are presently no common standard definition of what constitutes a PFAS (either domestically or internationally). There are presently significant on-going discussions looking to establish the suite of chemistries that should be characterized as PFASs, given that not all fluorinated chemistries should be considered as equivalent from an environmental and/or human health perspective. However, this work is on-going, including the present activities being conducted by the Government of Canada in support of a State of Science Report on PFAS in Canada, presently scheduled to be published in 2023.
- Regardless of these on-going discussions, ECCC appears to already be focused on proactively acting on PFASs in certain products – which is resulting in certain challenges for our members, despite our industry’s on-going engagement to look to identify viable substitution candidates and engage with responsible reformulation investments in relation to these ingredients.
- Moving forward, CA members are encouraged to examine your ingredient and product portfolios to identify any fluorinated chemistries of interest and undertake the following compliance due diligence:
- Consider whether or not any of your ingredients/products comprise PFASs that may be in scope of the 2012 Prohibition of Certain Toxic Substances (PCTS) Regulations (PFOA, PFOS, LC-PFCAs) [Schedule 2.1]
- Be aware that the PCTS Regulations are likely to evolve in 2022/2023. Stay tuned and understand how these regulatory updates might implicate your interests, moving forward
- Understand your New Substances Notification Regulations (NSNR) due diligence under CEPA in relation to these and other chemicals/polymers.
- Consider developing a regulatory assessment workplan for addressing considerations regarding any fluorinated chemistries (whether they are considered PFASs today – or that could ultimately be considered PFASs in the future) within your portfolio
Any questions regarding PFASs? Interested in a more in-depth briefing regarding these developments and our strategic approach on this issue? Don’t hesitate to reach out to your CA Canada Science and Regulatory Team (regulatory@cosmeticsalliance.ca)!